Fraiya launches lankmark NHS trial of AI-enabled pregnancy ultrasounds
London, UK – Tuesday 9th September 2025: Fraiya, a UK-based femtech startup transforming pregnancy ultrasound with AI, has announced a pivotal set of milestones, including NHS clinical evaluation, regulatory approval and pre-seed round, signalling its emergence as a serious player in maternal health innovation.
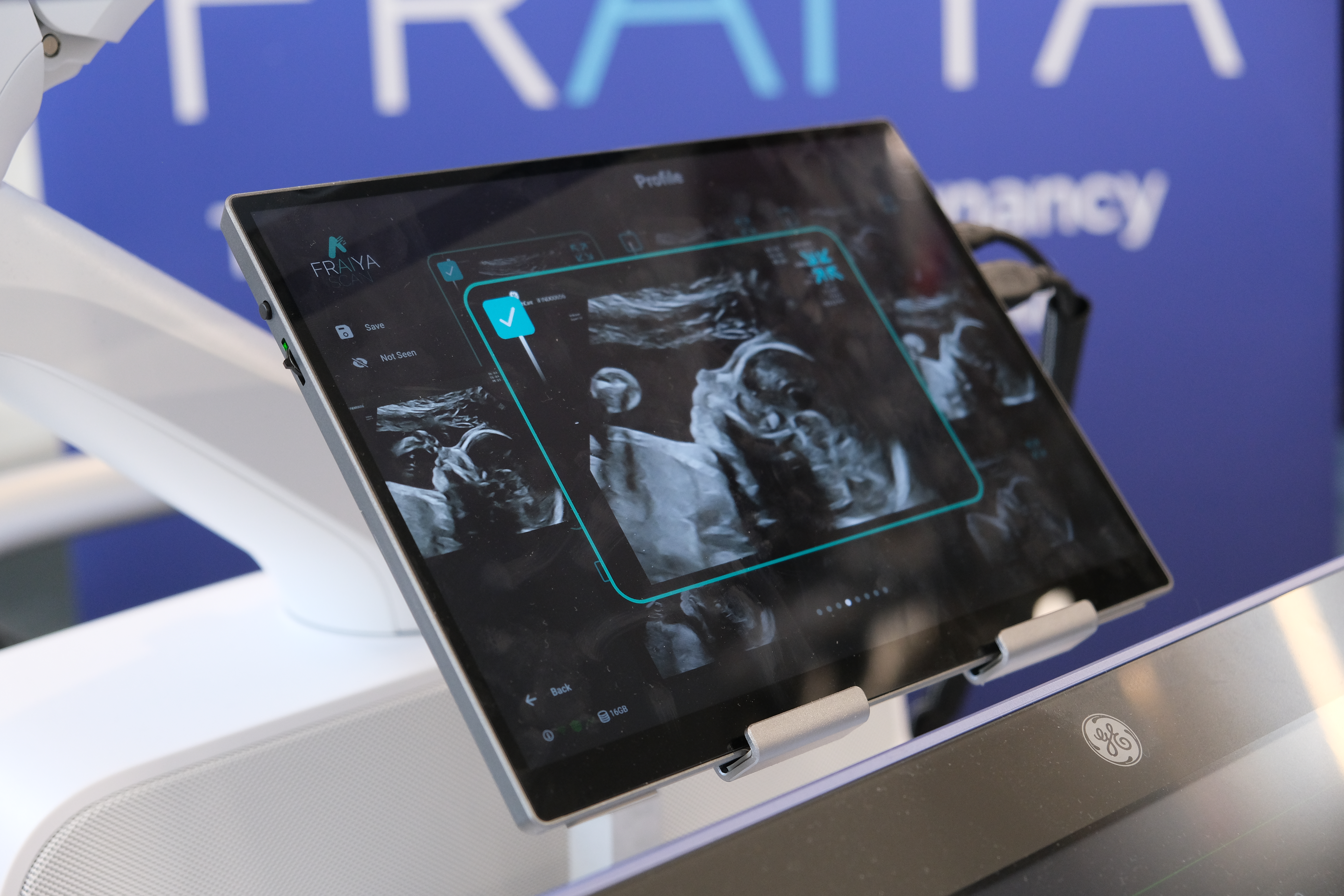
The company’s core product, FraiyaScan, supports clinicians during the 20-week anomaly scan by automating real-time image acquisition, quality checks, measurements, and clinical reporting assistance. Built from within the NHS and co-designed with sonographers and fetal medicine specialists, the tool integrates seamlessly into existing scanning workflows and ultrasound infrastructure, offering real-time support without disruption.

Team Fraiya (left to right): Prof Reza Razavi (CEO); Dr Jacqueline Matthew (CMO); Dr Alfonso Farruggia (Head of Engineering); Dr Sam Budd (CTO)
A landmark NHS trial for AI in obstetric imaging
Fraiya, working with King’s College London, is launching a prospective multi-centre randomised controlled trial of FraiyaScan, alongside an AI-assisted second review service, for more than 9,500 pregnant women across four NHS hospitals, one of the first large trials looking at clinical and health economic evaluation of an AI imaging tool.
Funded by the UK’s National Institute for Health and Care Research, the trial goes beyond performance metrics, measuring cost-effectiveness, workflow efficiency, and impact on workforce and patient experience. It addresses a critical gap in the literature; while AI is rapidly entering imaging, evidence for its impact on real-world healthcare remains virtually absent.
CE Marking under EU MDR
FraiyaScan is now a CE-marked Class IIa medical device, certified under the EU Medical Device Regulation (MDR) by Scarlet NB.
The company’s regulatory submission was led internally by Fraiya’s CTO Dr Sam Budd, with support from Jacqueline Beddoe-Rosendo and the Medical Engineering Quality and Regulatory Team at King’s, ensuring clinical safety and scalability from day one. This milestone enables commercial use in hospitals and clinics across the UK and EU, and demonstrates Fraiya’s commitment to clinical rigour and market readiness.
Pre-seed round to accelerate growth
The company has closed its pre-seed round, with a total of £3.5m in funding from grants, awards, VC and angel investment since October 2024. Backed by RAW Ventures, Cedars-Sinai Ventures, and a syndicate of Healthtech and AI-focused angel investors, the capital will fund:
- Clinical deployment across the UK and EU
- Additional regulatory submissions and approvals, including to the FDA
- Expansion into adjacent product lines
Backed by clinical and academic R&D
Fraiya originated from the iFIND project at King’s College London and Imperial College, a £10m EPSRC and Welcome Trust innovation grant at one of Europe’s leading Imaging Research Centres. The founding team combines deep technical and clinical expertise in AI, imaging science, digital and maternofetal health.
Fraiya is the alumnus of two medtech venture accelerators whose goal is to build AI that fits clinical workflow; the London Institute for Healthcare Engineering, and Cedars-Sinai Accelerator in Los Angeles.
Professor Razavi, Friaya’s CEO and chief investigator of the trial, said: “We see this trial as a turning point. It’s not just about proving our AI tools work, it’s about proving they add value to the health system.
“As a clinician who looks after babies with congenital problems, I see the difference between those who are diagnosed in pregnancy and get planned care with parents who are fully informed and prepared for what’s to come, and those who unfortunately were not picked up during the pregnancy scans, who arrive at our hospital very unwell and without a diagnosis, with very anxious parents, and have a more difficult journey.
“Fraiya’s mission is to address this problem of a lack of diagnosis during pregnancy, so all parents are aware of congenital problems with their babies, and babies are given the best care right from birth.”
Dr Jackie Matthew, Fraiya’s chief medical officer and clinical academic sonographer, added: “We’re focused on leveraging the unique capabilities of ultrasound and developing solutions to make it smarter, faster, and more reliable, with clinicians at the centre of that transformation.
“This trial will assess the effectiveness of FraiyaScan in real world conditions. Importantly, the frontline staff and patient feedback will help us to understand the acceptability of the technology, where time-pressured scans, staffing gaps, and service variability, that can affect outcomes, may also impact the performance and adoption of AI-based innovations.”
Dr Samuel Budd, Fraiya’s chief technology officer, added: “Excited to be building at the intersection of AI, clinical practice, and product development at Fraiya. With CE marking achieved, a trial on the horizon, and our pre-seed round complete, we’re moving fast toward real-world impact in prenatal care.”
Full campaign assets, including further images, available upon request from Fraiya Press Team
← Back to News